what does it mean for a membrane to be selectively permeable
15.three: Membrane Transport with Selective Permeability
- Folio ID
- 8531
Transport across the membrane
Design challenge problem and subproblems
Full general Problem: The jail cell membrane must simultaneously human action equally a bulwark between "IN" and "OUT" and control specifically which substances enter and exit the prison cell and how quickly and efficiently they practice and so.
Subproblems: The chemical properties of molecules that must enter and leave the cell are highly variable. Some subproblems associated with this are: (a) Big and small molecules or collections of molecules must exist able to pass beyond the membrane. (b) Both hydrophobic and hydrophilic substances must have admission to transport. (c) Substances must be able to cross the membrane with and against concentration gradients. (d) Some molecules look very similar (east.g. Na+ and M+) but transport mechanisms must still be able to distinguish between them.
Energy story perspective
Transport beyond a membrane tin exist considered from an energy story perspective; it is a process subsequently all. For example, at the first of the procedure a generic substance X may be either on the inside or outside of the cell. At the end of the procedure, the substance volition be on the opposite side from which it started.
due east.1000. Ten(in) ---> 10(out),
where in and out refer to within the cell and exterior the cell, respectively.
At the offset the matter in the system might exist a very complicated collection of molecules within and outside of the cell but with i molecule of X more than within the cell than out. At the stop, there is one more molecule of X on the outside of the cell and i less on the within. The energy in the system at the beginning is stored largely in the molecular structures and their motions and in electric and chemic concentration imbalances beyond the cell membrane. The ship of Ten out of the cell will not change the energies of the molecular structures significantly but it will change the energy associated with the imbalance of concentration and or charge across the membrane. That is the transport will, like all other reactions, exist either exergonic or endergonic. Finally, some mechanism or sets of mechanisms of ship will demand to exist described.
Selective permeability
One of the great wonders of the prison cell membrane is its power to regulate the concentration of substances inside the jail cell. These substances include: ions such every bit Catwo+, Na+, K+, and Cl–; nutrients including sugars, fatty acids, and amino acids; and waste products, especially carbon dioxide (CO2), which must leave the prison cell.
The membrane's lipid bilayer structure provides the first level of control. The phospholipids are tightly packed, and the membrane has a hydrophobic interior. This structure alone creates what is known as a selectively permeable barrier, one that only allows substances meeting certain physical criteria to laissez passer through it. In the example of the prison cell membrane, only relatively small, nonpolar materials can move through the lipid bilayer at biologically relevant rates (think, the lipid tails of the membrane are nonpolar).
Selective permeability of the prison cell membrane refers to its ability to differentiate between different types of molecules, only allowing some molecules through while blocking others. Some of this selective property stems from the intrinsic diffusion rates for different molecules beyond a membrane. A 2nd factor affecting the relative rates of movement of various substances across a biological membrane is activity of various protein-based membrane transporters, both passive and active, that will exist discussed in more particular in subsequent sections. First, we have on the notion of intrinsic rates of diffusion across the membrane.
Relative permeability
The fact that different substances might cantankerous a biological membrane at dissimilar rates should exist relatively intuitive. There are differences in the mosaic limerick of membranes in biology and differences in the sizes, flexibility, and chemical properties of molecules so it stands to reason that the permeability rates vary. Information technology is a complicated mural. The permeability of a substance across a biological membrane can be measured experimentally and the rate of movement across a membrane tin can exist reported in what are known equally membrane permeability coefficients.
Membrane permeability coefficients
Below, a diversity of compounds are plotted with respect to their membrane permeability coefficients (MPC) every bit measured against a elementary biochemical approximation of a real biological membrane. The reported permeability coefficient for this organization is the rate at which simple improvidence through a membrane occurs and is reported in units of centimeters per second (cm/s). The permeability coefficient is proportional to the division coefficient and is inversely proportional to the membrane thickness.
It is important that y'all are able to read and translate the diagram below. The larger the coefficient, the more permeable the membrane is to the solute. For case, hexanoic acid is very permeable, a MPC of 0.9; acetic acid, h2o, and ethanol have MPCs between 0.01 and 0.001, and they are less permeable than hexanoic acrid. Where as ions, such as sodium (Na+), have an MPC of ten-12, and cross the membrane at a insufficiently wearisome rate.

While at that place are certain trends or chemical properties that tin be roughly associated with different compound permeability (small thing go through "fast", big things "slowly", charged things not at all etc.), we caution against over-generalizing. The molecular determinants of membrane permeability are complicated and involve numerous factors including: the specific composition of the membrane, temperature, ionic limerick, hydration; the chemical properties of the solute; the potential chemical interactions betwixt the solute in solution and in the membrane; the dielectric properties of materials; and the energy trade-offs associated with moving substances into and out of various environments. Then, in this form, rather than try to utilise "rules" and attempt to develop too many arbitrary "cut-offs", nosotros will strive to develop a general sense of some properties that can influence permeability and leave the consignment of accented permeability to experimentally reported rates. In addition, nosotros will also try to minimize the use of vocabulary that depends on a frame of reference. For instance, saying that compound A diffuses "quickly" or "slowly" across a bilayer only means something if the terms "quickly" or "slowly" are numerically divers or the biological context is understood.
Energetics of transport
All substances that move through the membrane practise so by one of two general methods, which are categorized based on whether or not the transport procedure is exergonic or endergonic. Passive send is the exergonic movement of substances beyond the membrane. In contrast, active transport is the endergonic movement of substances across the membrane that is coupled to an exergonic reaction.
Passive transport
Passive ship does not require the prison cell to expend energy. In passive transport, substances move from an expanse of higher concentration to an area of lower concentration, downward their concentration gradient . Depending on the chemical nature of the substance, unlike processes may exist associated with passive transport.
Improvidence
Diffusion is a passive procedure of transport. A single substance tends to move from an area of high concentration to an area of depression concentration until the concentration is equal across a infinite. You are familiar with improvidence of substances through the air. For example, recall about someone opening a bottle of ammonia in a room filled with people. The ammonia gas is at its highest concentration in the canteen; its lowest concentration is at the edges of the room. The ammonia vapor will diffuse, or spread away, from the bottle; gradually, more and more people will smell the ammonia as it spreads. Materials move inside the prison cell's cytosol by diffusion, and certain materials movement through the plasma membrane by diffusion.
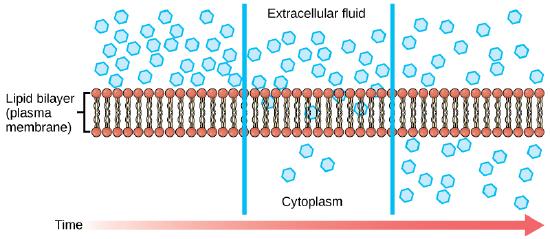
Factors that touch diffusion
If unconstrained, molecules will move through and explore infinite randomly at a charge per unit that depends on their size, their shape, their environment, and their thermal energy. This type of movement underlies the deviating movement of molecules through whatsoever medium they are in. The absence of a concentration gradient does not hateful that this motility will stop, just that there may be no net movement of the number of molecules from one area to another, a condition known as dynamic equilibrium.
Factors influencing diffusion include:
- Extent of the concentration gradient: The greater the difference in concentration, the more than rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Shape, size and mass of the molecules diffusing: Large and heavier molecules movement more than slowly; therefore, they lengthened more than slowly. The reverse is typically true for smaller, lighter molecules.
- Temperature: Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion. Lower temperatures decrease the energy of the molecules, thus decreasing the rate of diffusion.
- Solvent density: As the density of a solvent increases, the rate of diffusion decreases. The molecules dull down because they have a more difficult time getting through the denser medium. If the medium is less dumbo, rates of diffusion increment. Since cells primarily use diffusion to move materials inside the cytoplasm, whatever increase in the cytoplasm's density will decrease the rate at which materials motility in the cytoplasm.
- Solubility: As discussed earlier, nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion.
- Surface area and thickness of the plasma membrane: Increased surface area increases the rate of improvidence, whereas a thicker membrane reduces it.
- Altitude traveled: The greater the altitude that a substance must travel, the slower the charge per unit of diffusion. This places an upper limitation on cell size. A large, spherical jail cell will die because nutrients or waste matter cannot accomplish or leave the center of the prison cell, respectively. Therefore, cells must either exist small in size, every bit in the instance of many prokaryotes, or be flattened, as with many single-celled eukaryotes.
Facilitated transport
In facilitated transport, also chosen facilitated diffusion, materials diffuse across the plasma membrane with the assistance of membrane proteins. A concentration gradient exists that allows these materials to lengthened into or out of the cell without expending cellular energy. In the case that the materials are ions or polar molecules (compounds that are repelled by the hydrophobic parts of the cell membrane), facilitated transport proteins help shield these materials from the repulsive force of the membrane, allowing them to lengthened into the cell.
Note: possible discussion
Compare and contrast passive diffusion and facilitated diffusion.
Channels
The integral proteins involved in facilitated transport are collectively referred to equally transport proteins, and they function as either channels for the cloth or carriers. In both cases, they are transmembrane proteins. Different channel proteins have different transport properties. Some take evolved to have very high specificity for the substance that is being transported while others send a variety of molecules sharing some common characteristic(s). The interior "passageway" of aqueduct proteins take evolved to provide a depression energetic bulwark for send of substances beyond the membrane through the complementary arrangement of amino acid functional groups (of both backbone and side-chains). Passage through the channel allows polar compounds to avoid the nonpolar central layer of the plasma membrane that would otherwise dull or foreclose their entry into the prison cell. While at whatever one time significant amounts of h2o crosses the membrane both in and out, the rate of individual water molecule ship may not be fast enough to accommodate to changing ecology atmospheric condition. For such cases Nature has evolved a special course of membrane proteins called aquaporins that permit water to pass through the membrane at a very high rate.

Channel proteins are either open at all times or they are "gated." The latter controls the opening of the channel. Various mechanisms may be involved in the gating mechanism. For instance, the zipper of a specific ion or small molecule to the aqueduct protein may trigger opening. Changes in local membrane "stress" or changes in voltage across the membrane may also be triggers to open or close a channel.
Different organisms and tissues in multicellular species express different sets of aqueduct proteins in their membranes depending on the environments they alive in or specialized function they play in an organisms. This provides each blazon of jail cell with a unique membrane permeability contour that is evolved to complement its "needs" (notation the anthropomorphism). For example, in some tissues, sodium and chloride ions pass freely through open channels, whereas in other tissues a gate must be opened to let passage. This occurs in the kidney, where both forms of channels are found in different parts of the renal tubules. Cells involved in the manual of electric impulses, such equally nerve and musculus cells, take gated channels for sodium, potassium, and calcium in their membranes. Opening and closing of these channels changes the relative concentrations on opposing sides of the membrane of these ions, resulting a change in electrical potential across the membrane that lead to message propagation in the case of nerve cells or in muscle wrinkle in the instance of muscle cells.
Carrier proteins
Another type of poly peptide embedded in the plasma membrane is a carrier protein. This aptly named poly peptide binds a substance and, in doing and so, triggers a change of its own shape, moving the bound molecule from the outside of the cell to its interior; depending on the gradient, the material may motion in the opposite management. Carrier proteins are typically specific for a single substance. This selectivity adds to the overall selectivity of the plasma membrane. The molecular-scale mechanism of role for these proteins remains poorly understood.

Carrier protein play an important role in the function of kidneys. Glucose, water, salts, ions, and amino acids needed by the body are filtered in one part of the kidney. This filtrate, which includes glucose, is and then reabsorbed in some other function of the kidney with the help of carrier proteins. Considering at that place are only a finite number of carrier proteins for glucose, if more glucose is nowadays in the filtrate than the proteins tin can handle, the excess is not reabsorbed and it is excreted from the body in the urine. In a diabetic individual, this is described as "spilling glucose into the urine." A different group of carrier proteins chosen glucose ship proteins, or GLUTs, are involved in transporting glucose and other hexose sugars through plasma membranes within the body.
Channel and carrier proteins send cloth at unlike rates. Channel proteins transport much more than quickly than do carrier proteins. Channel proteins facilitate improvidence at a charge per unit of tens of millions of molecules per second, whereas carrier proteins work at a rate of a thousand to a 1000000 molecules per second.
Active send
Agile transport mechanisms require the utilise of the cell's energy, unremarkably in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient—that is, if the concentration of the substance within the prison cell is greater than its concentration in the extracellular fluid (and vice versa)—the prison cell must apply energy to motion the substance. Some active send mechanisms move small-molecular weight materials, such as ions, through the membrane. Other mechanisms send much larger molecules.
Moving against a slope
To move substances against a concentration or electrochemical gradient, the cell must utilise free energy. This energy is harvested from ATP generated through the prison cell's metabolism. Active transport mechanisms, collectively called pumps, work against electrochemical gradients. Small-scale substances constantly laissez passer through plasma membranes. Active transport maintains concentrations of ions and other substances needed by living cells in the confront of these passive movements. Much of a cell'south supply of metabolic free energy may be spent maintaining these processes. (Most of a red blood cell's metabolic energy is used to maintain the imbalance betwixt exterior and interior sodium and potassium levels required by the prison cell.) Because active send mechanisms depend on a cell's metabolism for energy, they are sensitive to many metabolic poisons that interfere with the supply of ATP.
Two mechanisms exist for the transport of small-molecular weight material and modest molecules. Primary agile transport moves ions across a membrane and creates a divergence in charge across that membrane, which is straight dependent on ATP. Secondary agile ship describes the motion of textile that is due to the electrochemical slope established past primary agile ship that does not directly require ATP.
Carrier proteins for active transport
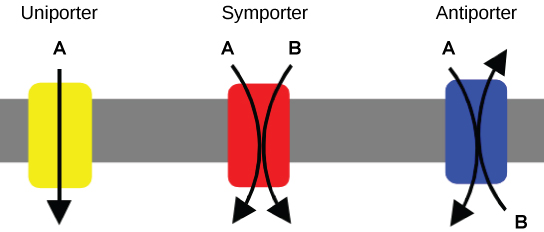
An important membrane adaption for active transport is the presence of specific carrier proteins or pumps to facilitate movement: there are three types of these proteins or transporters. A uniporter carries one specific ion or molecule. A symporter carries 2 different ions or molecules, both in the same management. An antiporter also carries two unlike ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules like glucose. These three types of carrier proteins are also plant in facilitated improvidence, but they do not crave ATP to piece of work in that process. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-Yard+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca2+ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
Master active transport
In principal active transport, the energy is often - though not exclusively - derived directly from the hydrolysis of ATP. Ofttimes, principal active transport, such as that shown below, which functions to transport sodium and potassium ions allows secondary active transport to occur (discussed in the section below). The second ship method is all the same considered agile because it depends on the utilise of free energy from the principal transport.
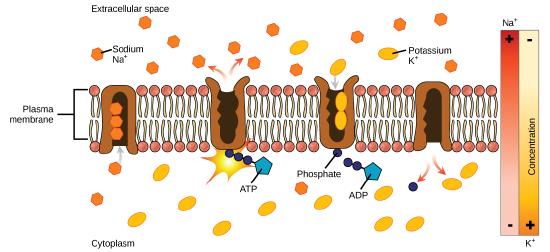
One of the nigh important pumps in beast cells is the sodium-potassium pump (Na+-1000+ ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na+and K+) in living cells. The sodium-potassium pump moves K+ into the prison cell while moving Na+ out at the same time, at a ratio of three Na+ for every ii Chiliad+ ions moved in. The Na+-1000+ATPase exists in two forms depending on its orientation to the interior or exterior of the jail cell and its affinity for either sodium or potassium ions. The process consists of the post-obit six steps.
- With the enzyme oriented towards the interior of the cell, the carrier has a high affinity for sodium ions. Three ions bind to the protein.
- ATP is hydrolyzed by the poly peptide carrier and a low-energy phosphate group attaches to it.
- As a result, the carrier changes shape and re-orients itself towards the exterior of the membrane. The protein'due south affinity for sodium decreases and the three sodium ions leave the carrier.
- The shape change increases the carrier's analogousness for potassium ions, and two such ions attach to the protein. Subsequently, the low-energy phosphate group detaches from the carrier.
- With the phosphate group removed and potassium ions attached, the carrier poly peptide repositions itself towards the interior of the cell.
- The carrier poly peptide, in its new configuration, has a decreased affinity for potassium, and the two ions are released into the cytoplasm. The protein now has a higher analogousness for sodium ions, and the process starts once more.
Several things have happened as a result of this procedure. At this point, there are more sodium ions outside of the prison cell than inside and more potassium ions inside than out. For every three ions of sodium that motion out, two ions of potassium move in. This results in the interior being slightly more negative relative to the exterior. This divergence in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Link to learning
Visit the site to see a simulation of agile transport in a sodium-potassium ATPase.
Secondary active transport (co-transport)
Secondary active transport brings sodium ions, and peradventure other compounds, into the cell. As sodium ion concentrations build outside of the plasma membrane because of the action of the primary active transport process, an electrochemical slope is created. If a channel protein exists and is open up, the sodium ions volition be pulled through the membrane. This movement is used to transport other substances that can attach themselves to the transport protein through the membrane. Many amino acids, as well as glucose, enter a cell this style. This secondary process is also used to store high energy hydrogen ions in the mitochondria of constitute and animal cells for the production of ATP. The potential free energy that accumulates in the stored hydrogen ions is translated into kinetic energy equally the ions surge through the channel protein ATP synthase, and that energy is used to convert ADP into ATP.

Osmosis
Osmosis is the movement of water through a semipermeable membrane according to the concentration gradient of water across the membrane, which is inversely proportional to the concentration of solutes. While improvidence transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the diffusion of solutes in the water. Non surprisingly, the aquaporins that facilitate water motion play a big part in osmosis, most prominently in red blood cells and the membranes of kidney tubules.
Mechanism
Osmosis is a special example of diffusion. Water, similar other substances, moves from an expanse of high concentration to one of low concentration. An obvious question is what makes h2o movement at all? Imagine a beaker with a semipermeable membrane separating the two sides or halves. On both sides of the membrane the water level is the same, but there are different concentrations of a dissolved substance, or solute, that cannot cross the membrane (otherwise the concentrations on each side would be balanced by the solute crossing the membrane). If the volume of the solution on both sides of the membrane is the aforementioned, simply the concentrations of solute are dissimilar, and so there are dissimilar amounts of water, the solvent, on either side of the membrane.
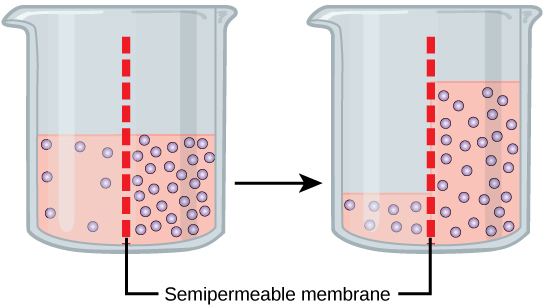
To illustrate this, imagine 2 full glasses of water. Ane has a single teaspoon of sugar in it, whereas the second 1 contains one-quarter cup of carbohydrate. If the total volume of the solutions in both cups is the same, which cup contains more water? Considering the large amount of sugar in the second loving cup takes upward much more space than the teaspoon of sugar in the first cup, the beginning cup has more water in it.
Returning to the beaker instance, recall that it has a mixture of solutes on either side of the membrane. A principle of diffusion is that the molecules move effectually and will spread evenly throughout the medium if they can. However, just the material capable of getting through the membrane will diffuse through it. In this case, the solute cannot diffuse through the membrane, but the water can. Water has a concentration gradient in this arrangement. Thus, h2o will diffuse downwards its concentration slope, crossing the membrane to the side where it is less concentrated. This improvidence of water through the membrane—osmosis—will continue until the concentration slope of water goes to zero or until the hydrostatic pressure of the h2o balances the osmotic pressure. Osmosis proceeds constantly in living systems.
Tonicity
Tonicity describes how an extracellular solution can change the volume of a jail cell past affecting osmosis. A solution's tonicity often direct correlates with the osmolarity of the solution. Osmolarity describes the total solute concentration of the solution. A solution with depression osmolarity has a greater number of h2o molecules relative to the number of solute particles; a solution with high osmolarity has fewer water molecules with respect to solute particles. In a situation in which solutions of two different osmolarities are separated by a membrane permeable to water, though not to the solute, water will move from the side of the membrane with lower osmolarity (and more h2o) to the side with higher osmolarity (and less water). This effect makes sense if you remember that the solute cannot move across the membrane, and thus the only component in the system that tin move—the water—moves along its ain concentration slope. An of import stardom that concerns living systems is that osmolarity measures the number of particles (which may be molecules) in a solution. Therefore, a solution that is cloudy with cells may take a lower osmolarity than a solution that is clear if the second solution contains more dissolved molecules than there are cells.
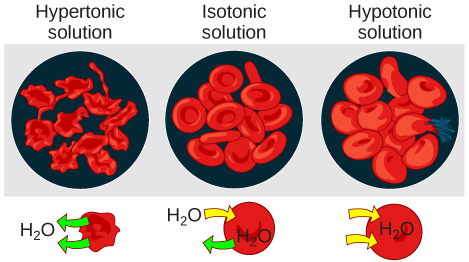
Hypotonic solutions
Three terms—hypotonic, isotonic, and hypertonic—are used to chronicle the osmolarity of a cell to the osmolarity of the extracellular fluid that contains the cells. In a hypotonic situation, the extracellular fluid has lower osmolarity than the fluid within the cell, and water enters the prison cell (in living systems, the point of reference is always the cytoplasm, so the prefix hypo- means that the extracellular fluid has a lower concentration of solutes, or a lower osmolarity, than the cell cytoplasm). It also means that the extracellular fluid has a higher concentration of water in the solution than does the cell. In this state of affairs, h2o will follow its concentration gradient and enter the prison cell.
Hypertonic solutions
As for a hypertonic solution, the prefix hyper- refers to the extracellular fluid having a higher osmolarity than the cell's cytoplasm; therefore, the fluid contains less water than the prison cell does. Because the cell has a relatively college concentration of h2o, water will leave the cell.
Isotonic solutions
In an isotonic solution, the extracellular fluid has the same osmolarity every bit the cell. If the osmolarity of the cell matches that of the extracellular fluid, in that location will be no net movement of water into or out of the cell, although h2o will still motion in and out. Blood cells and found cells in hypertonic, isotonic, and hypotonic solutions take on characteristic appearances.
Connection
A doctor injects a patient with what the doctor thinks is an isotonic saline solution. The patient dies, and an autopsy reveals that many crimson blood cells have been destroyed. Do you recall the solution the doctor injected was really isotonic?
Link to learning
For a video illustrating the process of improvidence in solutions, visit this site.
Tonicity in living systems
In a hypotonic surround, water enters a cell, and the jail cell swells. In an isotonic condition, the relative concentrations of solute and solvent are equal on both sides of the membrane. There is no net water movement; therefore, at that place is no change in the size of the jail cell. In a hypertonic solution, water leaves a cell and the cell shrinks. If either the hypo- or hyper- condition goes to excess, the cell's functions go compromised, and the cell may be destroyed.
A cherry-red blood cell will outburst, or lyse, when it swells beyond the plasma membrane'southward adequacy to expand. Remember, the membrane resembles a mosaic, with discrete spaces between the molecules composing it. If the cell swells, and the spaces between the lipids and proteins become as well big, and the cell will break apart.
In contrast, when excessive amounts of water go out a ruby blood cell, the cell shrinks, or crenates. This has the effect of concentrating the solutes left in the prison cell, making the cytosol denser and interfering with diffusion inside the prison cell. The jail cell's ability to function will be compromised and may as well result in the expiry of the cell.
Diverse living things take ways of controlling the furnishings of osmosis—a mechanism called osmoregulation. Some organisms, such as plants, fungi, bacteria, and some protists, accept jail cell walls that surround the plasma membrane and foreclose jail cell lysis in a hypotonic solution. The plasma membrane can but expand to the limit of the cell wall, so the cell will not lyse. In fact, the cytoplasm in plants is always slightly hypertonic to the cellular environment, and h2o will ever enter a cell if water is available. This inflow of water produces turgor pressure, which stiffens the cell walls of the plant. In nonwoody plants, turgor pressure supports the plant. Conversely, if the establish is non watered, the extracellular fluid will become hypertonic, causing water to leave the prison cell. In this condition, the cell does not shrink because the prison cell wall is not flexible. However, the jail cell membrane detaches from the wall and constricts the cytoplasm. This is chosen plasmolysis. Plants lose turgor force per unit area in this condition and wilt.
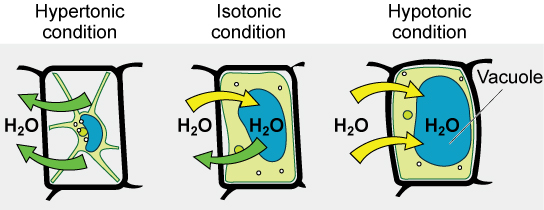
Tonicity is a business concern for all living things. For example, paramecia and amoebas, which are protists that lack cell walls, have contractile vacuoles. This vesicle collects backlog h2o from the cell and pumps it out, keeping the jail cell from bursting as it takes on water from its environs.
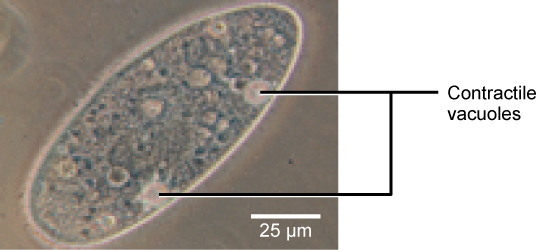
Figure 12 . A paramecium'due south contractile vacuole, here visualized using brilliant field light microscopy at 480x magnification, continuously pumps water out of the organism'due south body to continue it from bursting in a hypotonic medium. (credit: modification of work by NIH; scale-bar information from Matt Russell)
Many marine invertebrates accept internal salt levels matched to their environments, making them isotonic with the water in which they live. Fish, however, must spend approximately five per centum of their metabolic energy maintaining osmotic homeostasis. Freshwater fish live in an environment that is hypotonic to their cells. These fish actively take in salt through their gills and excrete diluted urine to rid themselves of backlog water. Saltwater fish live in the reverse surroundings, which is hypertonic to their cells, and they secrete salt through their gills and excrete highly concentrated urine.
In vertebrates, the kidneys regulate the amount of h2o in the torso. Osmoreceptors are specialized cells in the brain that monitor the concentration of solutes in the claret. If the levels of solutes increase beyond a certain range, a hormone is released that retards water loss through the kidney and dilutes the blood to safer levels. Animals likewise accept high concentrations of albumin, which is produced past the liver, in their claret. This protein is too large to pass easily through plasma membranes and is a major cistron in controlling the osmotic pressures applied to tissues.
Source: https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A:_Introductory_Biology_%28Easlon%29/Readings/15.3:_Membrane_Transport_with_Selective_Permeability

0 Response to "what does it mean for a membrane to be selectively permeable"
Post a Comment